Downstream Module Automation
Our client developed laboratory systems that can synthesize and purify pharmaceuticals using continuous-flow synthesis and formulation of Active Pharmaceutical Ingredients (APIs) in a compact, reconfigurable manufacturing platform. Continuous pharmaceutical production in compact modules enables drug manufacturing on-demand and at the location of need, resulting in substantially reduced lead times.
Using this approach, the systems transform shelf-stable raw materials into thousands of doses of a target drug within 24 hours. Change the precursor chemicals, change the connections between the reactor vessels, change the recipe, and the same system can make Benadryl today and Prozac tomorrow.
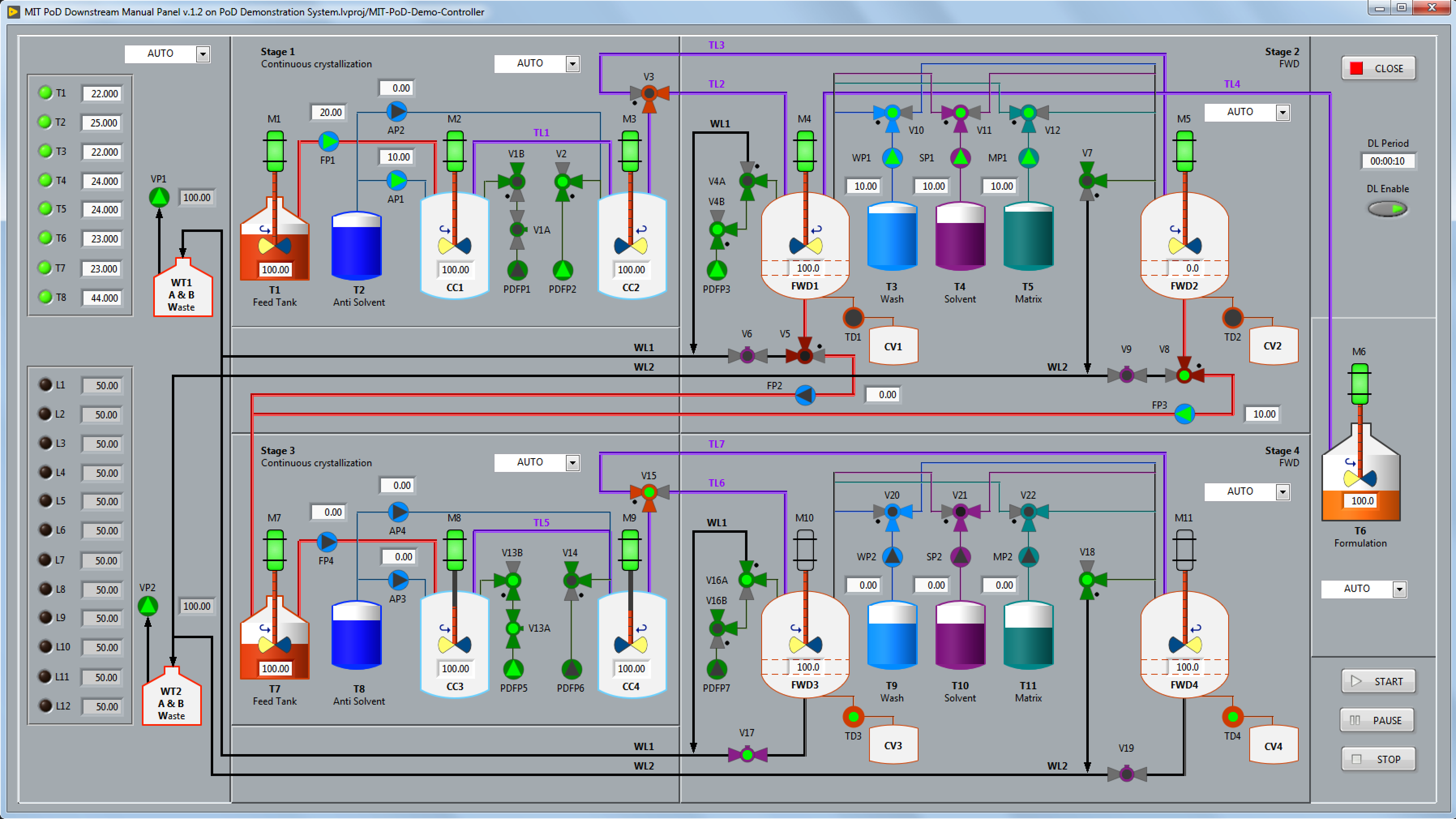
As part of the Pharmacy on Demand (PoD) Demonstration Phase, a new series of refrigerator-sized modules was designed and constructed to enable the synthesis, isolation, and formulation of multiple pharmaceutical compounds and enable the production of both liquids and tablet doses.
The systems had to demonstrate the synthesis and formulation of five different APIs:
- Diazepam (Valium)
- Diphenhydramine Hydrochloride (Benadryl)
- Ciprofloxacin Hydrochloride (Antibiotic)
- Lidocaine Hydrochloride (Xylocaine)
- Atropine Sulfate (Anticholinergic)
Eagle Lake developed the real-time systems architecture and LabVIEW software to automate the PoD Downstream module for the Demonstration phase. The combination of reconfigurability, the number of discrete control elements, and the unique challenges associated with continuous flow chemistry necessitated a new approach compared to a conventional batch processing methods.
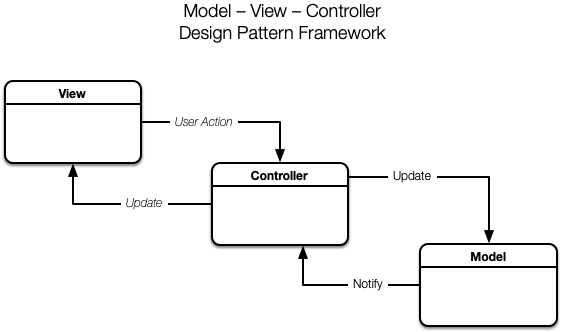
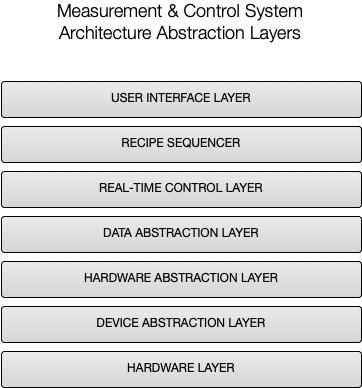
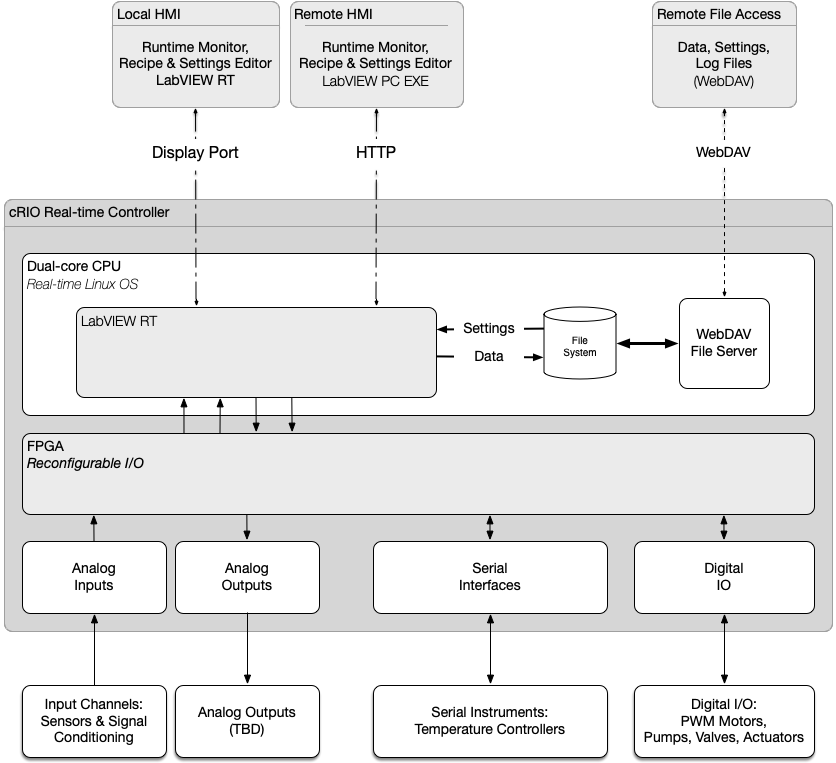
The delivered system architecture is based upon Eagle Lake’s proven LabVIEW RT + FPGA software architecture, which utilizes a layered Model-View-Controller (MVC) framework running on an NI cRIO embedded controller.
The MODEL in the MVC framework encapsulates state of the hundreds of system IO endpoints, and is implemented in a hierarchy of layered, multi-threaded subsystems. Our unique Hardware Abstraction Layer is composed of independent device drivers to monitor and control the hardware in a consistent way. Access to the hardware is further abstracted from the VIEW and the CONTROLLER by our Data Abstraction Layer, providing a hardware independent representation of the Model.
For the VIEW, Eagle Lake created custom controls and indicators to build Human Machine Interface (HMI) panels that replicate the process flow diagram, including color-coded graphics to view and/or control the state of the valves, pumps, motors, and liquid tanks. The user interface panels use our proprietary “no wiring” view-controller API which connects to the MODEL via the Data Abstraction Layer.
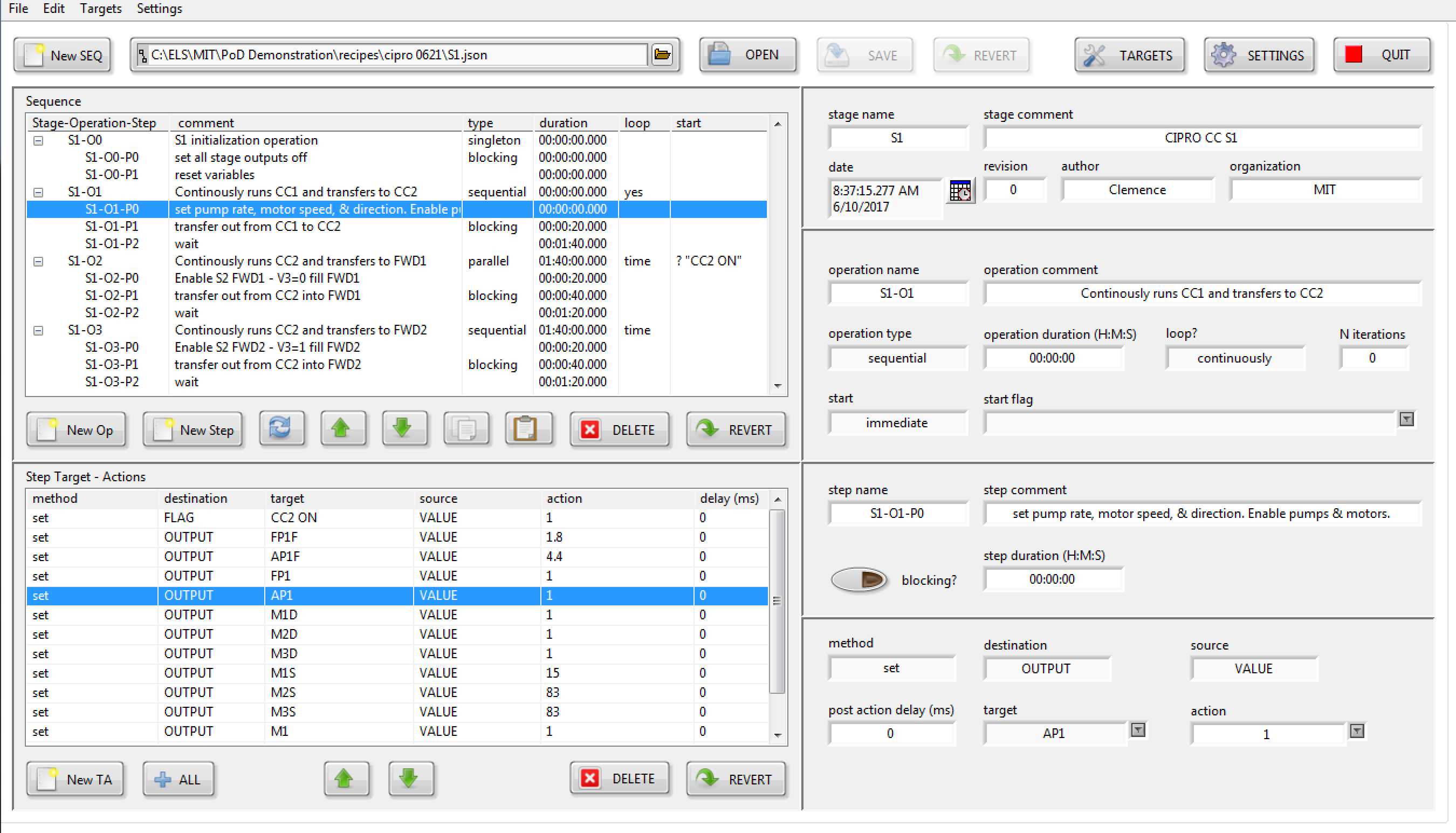
For the PoD CONTROLLER, Eagle Lake created a novel continuous-flow recipe sequencer to simultaneously control each successive processing stage to synthesize and purify the active ingredient.
In contrast to traditional batch processing systems that follow a linear, step-by-step sequence of operations, the PoD process stages operate continuously and concurrently. Furthermore, since the same system can process many different kinds of APIs, a fixed state machine approach was impractical.
Utilizing a custom recipe sequence editor, a chemist can create recipes for each API that specify the parallel processing operations for each stage and the precise steps involved in each operation.
When execution begins, the recipe sequencer employs a dynamic recursive-reentrant “Factory” design pattern. This pattern recursively launches an arbitrary number of stage sequencers, each of which can subsequently recursively launch an arbitrary number of operation sequencers. Consequently, all of the sequencers are executing concurrently.
Semaphores are employed within each stage to guarantee exclusive access to hardware by a single operations sequencer until its completion.
Each operation comprises an array of Steps, each of which contains a data layer destination (Target) and source (Action). For instance, to actuate a valve, the action value of “1” is sent to the named control output via the data layer.
Since all recipe sequencer I/O interactions are routed through the data abstraction layer, the recipe remains independent of the hardware and implementation.
Furthermore, by incorporating a simulation feature into the device abstraction layer, we built the entire PoD Downstream MVC application as a PC executable running in simulation mode on the chemist’s laptop. This enables them to independently create, edit, execute, test, debug, and validate an entire multi-stage recipe for each API on their PC, before deploying it to run on the embedded controller target system, controlling the hardware with chemicals.
Our client successfully demonstrated the downstream module to the end customer, processing all five APIs on schedule.